IT_Version
Since a few decades selective cellular and tissutal targeting of drugs has become a popular goal, both to overcame general toxicity issues and to increase effucacy at the site of action. Liposomal constructs represent a pursued research avenue, and our NEVERMIND project is an excellent example.
To this regard, we were stimulated by our role in NEVERMIND as designers and producers of modified phospholipids capable, once copolymerized in liposomes, to provide sensitivity to pathological brain microenvironments – i.e., matrix metalloprotease-rich glioblastoma and Alzheimer’s biosamples. We partitioned the collected material based on the specific pathology-connected stimuli providing the controlled dismantling of modified liposomes and cargo release, and we transferred such material in a recently published review.1 A short synopsis, including a few examples, is reported below.
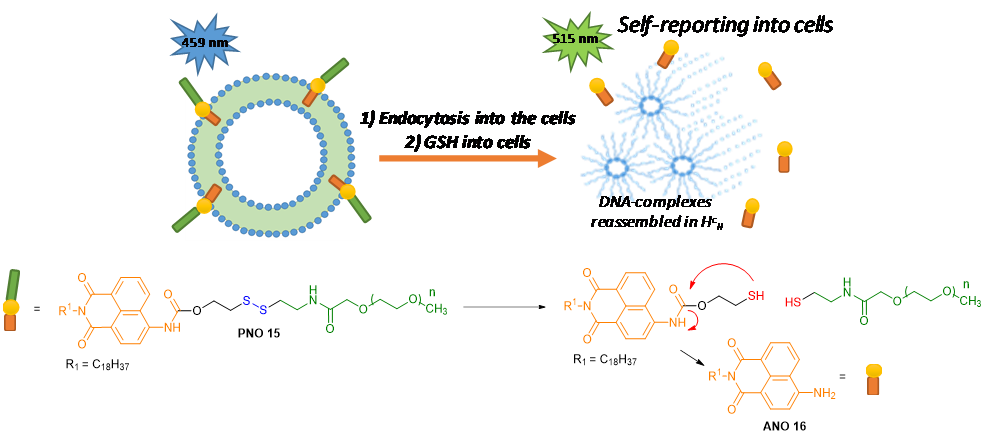
The first section deals with redox-sensitive liposomes. Oxidoreductions may induce the destabilization of liposomes and controlled cargo release either by locally changing charge or lipophilic content, or by causing the breakage of a redox-sensitive chemical bond, or by induced conformational changes of modified lipophilic copolymers; often an S-S bond is bioreduced, taking advantage of glutathione-GSH and of its reductase enzyme. As an example you see below a liposome, containing modified copolymers bearing an S-S bond which connects a fluorescent blue PNO group; after disulfide reduction and cleavage, the green fluorescent ANO compound is released to enable imaging studies.
Then we dealt with liposomi enzyme-sensitive liposomes, taking advantage of dysregulations (mostly overexpression) of one or more enzymes in pathological environments; for example, several proteases are overexpressed in tumor-affected tissues. The figure below shows a cholesterol-polyacrylic acid-uPA sequence construct, which is copolymerized in modified liposomes and stabilizes them in healthy tissues. Conversely, the over-activated uPA protease hydrolyzes the uPA peptide sequence in in several tumors, thus destabilizing the whole liposome and causing the controlled release of its cargo.
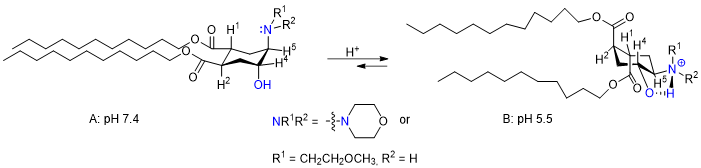
Another section deals with pH-sensitive liposomes, which modulate their stability and cargo release depending on the pH of the microenvironment of tumors, or of other pathologies characterized by pH lowering. A modified lipopeptide, built on a trans-cycloaminohexanol, can be copolymerized in its bis-equatorial conformation (left below) to yield a stable liposome at physiologic pH. Once the liposome reaches the slightly acidic pH of the tumor microenvironment, a strong hydrogen bond is created between the positively charged ammonium salt and the hydroxy group on the cycle, causing a transition to a diaxial lipophilic chain transition (right below) that destabilizes the whole liposome and causes its controlled dismantling / cargo release.
Finally, i hypoxia-sensitive liposomes are destabilized by poor oxygen content conditions typical in some tumors. The Figure below shows on the left a modified lipophilic construct, containing a stable azo bond;in hypoxic tumoral environments, the hypoxia-inducible factor 1 α (HIF-1 α) is activated and promotes the reductive cleavage of the azo bond (right below), once more leading to liposome destabilization and cargo release in a stimulus-depender manner.

1. Antoniou, A.I; Giofrè, S.; Seneci, P.; Passarella, D.; Pellegrino, S. Stimulus-responsive liposomes for biomedical applications. Drug Discov. Today 26 (2021), 1794-1824.
Page by Prof. Seneci – Università degli Studi di Milano, Partner of the NEVERMIND Consortium